Verzenio + hormone therapy
Learn about the dosing and efficacy of Verzenio + hormone therapy and how they work inside the body.
What is Verzenio?
Verzenio + hormone therapy is specifically for women with HR+, HER2–, node-positive early breast cancer with a high chance of returning, as determined by a healthcare provider.
Verzenio in addition to your hormone therapy (tamoxifen or an aromatase inhibitor) can reduce your risk of recurrence vs hormone therapy alone.
Verzenio was studied in a clinical study of 5637 adults with HR+, HER2–, node-positive early breast cancer with a high chance of returning who had previously received primary treatment. In this study, 2808 patients were treated with Verzenio plus standard hormone therapy for 2 years, and then hormone therapy alone; 2829 patients received standard hormone therapy alone. The approval was based on a smaller group of 5120 patients from this study.
The recommended dose of Verzenio in combination with hormone therapy is 150 mg by mouth twice a day, taken once in the morning and once at night.
- Each pack contains your dose of 150-mg tablets for 7 days (14 individual 150-mg tablets)
- Multiple tablet strengths (50 mg, 100 mg, and 150 mg) allow your doctor to make dose adjustments based on how you tolerate the drug
- No matter what dose is prescribed, you will take 1 tablet, 2 times a day, unless your doctor tells you otherwise
Finally, treatment is moving forward, just like you. Verzenio is the first and only treatment in over 15 years to reduce the risk of recurrence in HR+, HER2– high-risk early breast cancer, when added to hormone therapy.
Results with Verzenio + hormone therapy
The 4-year data on the risk reduction of Verzenio + hormone therapy vs hormone therapy alone is the longest study of its kind.
In this clinical study, Verzenio + hormone therapy was evaluated in people with HR+, HER2– early breast cancer with a high risk of recurrence based on extent of nodal involvement, tumor size, and tumor grade.

Reduced risk of recurrence
Verzenio helps kill cancer cells left behind after surgery, chemotherapy, and radiation to help prevent recurrence.
Verzenio + hormone therapy showed a 35% reduction in the risk of cancer returning compared with hormone therapy alone.
In a study, 85.5% of people taking Verzenio + hormone therapy were alive without their cancer returning vs 78.6% taking hormone therapy alone. Data was assessed at 48 months; the study is ongoing to see if there is a survival benefit.
In a 4-year evaluation, Verzenio + hormone therapy showed a lower risk of cancer recurrence beyond the 2-year treatment period compared to hormone therapy alone.
SELECT SAFETY INFORMATION
Verzenio may cause serious side effects, including:
Diarrhea is common with Verzenio, may be severe and may cause dehydration or infection. The most common time to develop diarrhea is during the first month of Verzenio treatment. If you develop diarrhea during treatment, your healthcare provider may tell you to temporarily stop taking it, stop your treatment, or decrease your dose. If you have any loose stools, start taking an antidiarrheal medicine (such as loperamide), drink more fluids, and tell your healthcare provider right away.
What makes Verzenio different?
Verzenio has been used to treat over 15,000 patients in the U.S. with certain types of HR+, HER2– metastatic breast cancer
and is FDA approved to treat patients with HR+, HER2–, node-positive, early breast cancer with a high risk of coming back, in combination with hormone therapy.
Verzenio is the first and only treatment of its kind that can further reduce the risk of recurrence
when combined with hormone therapy vs hormone therapy alone.
Verzenio is a tablet and not an infusion.
This means you do not need to go to your doctor's office for this treatment. However, you may need to see your doctor for other treatments or tests. You can take Verzenio in your own home, which could give you more flexibility in your daily life.
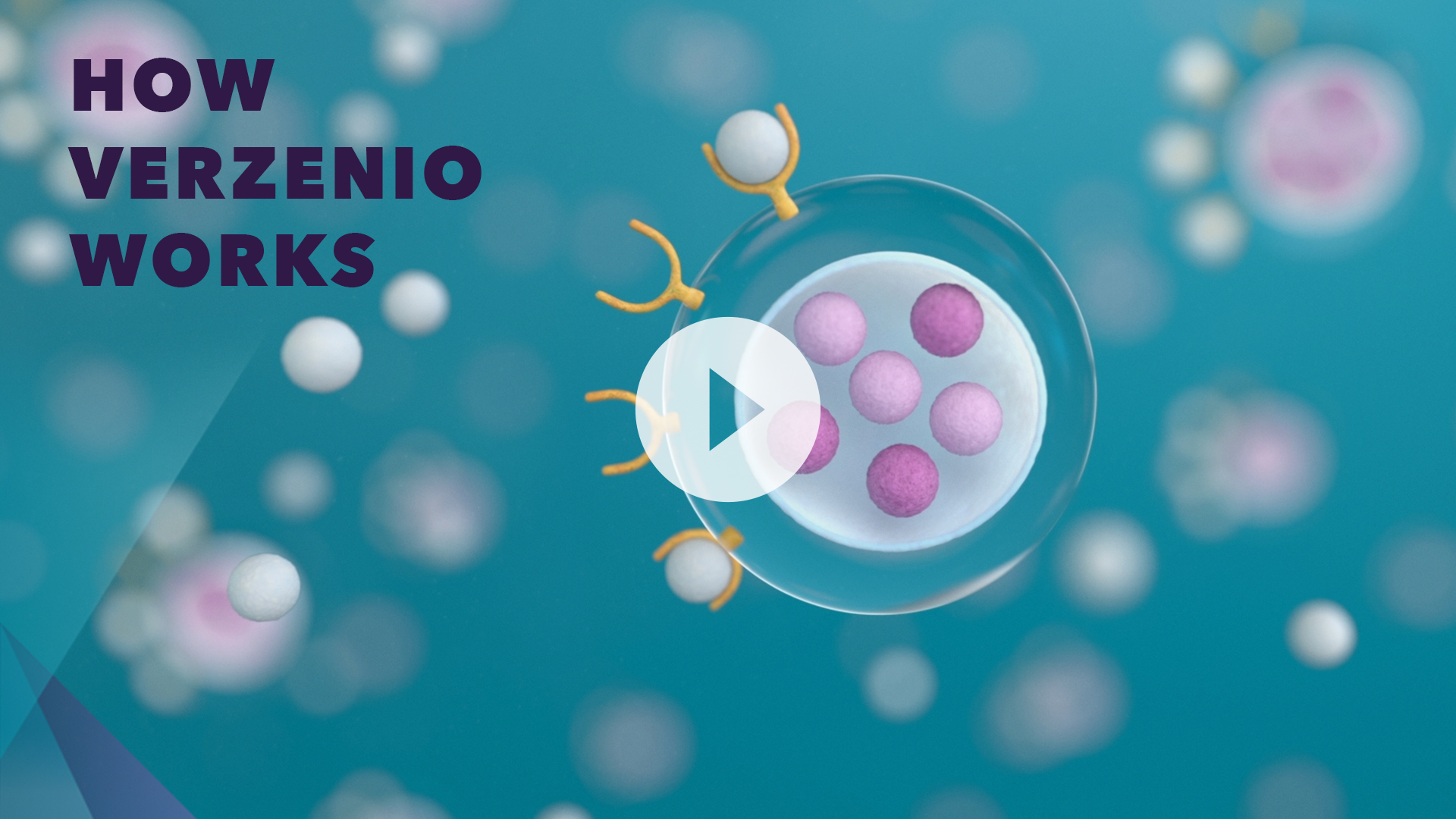
How Verzenio works
Verzenio is a CDK4 & 6 inhibitor used to treat HR+, HER2–, node-positive, early breast cancer with a high risk of coming back as determined by your doctor.*
Verzenio works to block specific proteins called CDK4 & CDK6.
CDK4 & 6 proteins help control how fast cells grow.
These proteins, along with hormones like estrogen, play an important role in the growth and division process of normal cells.
But if you have HR+, HER2– early breast cancer, CDK4 & 6 proteins become overactive, causing the cancer cells to grow and divide uncontrollably, which makes the tumor grow.
Verzenio is taken with hormone therapy to help prevent cancer recurrence after active treatment.
Hormone therapy works outside the cell to reduce estrogen that feeds the cancer.
Verzenio works inside the cell to block CDK4 & 6 activity and help stop the growth of cancer cells.† When Verzenio inhibits CDK4 & 6 in healthy cells, it can lead to side effects, some of which may be serious.
*Verzenio is taken after active therapy.
†Based on pre-clinical studies.
CDK4 & 6=cyclin-dependent kinases 4 and 6.
00:00-00:10
[The Verzenio abemaciclib 50 | 100 | 150 mg tablets logo is to the left of captions.]
Caption: Verzenio is a prescription medicine used:
- In combination with hormone therapy (tamoxifen or an aromatase inhibitor) to treat adults with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, early breast cancer with a high risk of coming back as determined by your healthcare provider.
It is not known if it is safe and effective in children.
Please read and listen to the Indication and Safety Summary following this video.
00:11-00:22
[The Verzenio abemaciclib 50 | 100 | 150 mg tablets logo is center screen.]
AVO: Being diagnosed with early breast cancer can be challenging for you and your loved ones. This video is here to help, so you can better understand your treatment and how Verzenio works in the body.
Caption: How Verzenio works
Caption: Please read and listen to the Indication and Safety Summary following this video.
00:23-00:38
[The captions fade out. Transition to an animated cell.]
AVO: Verzenio is a treatment for HR+, HER2–, node-positive early breast cancer with a high risk of coming back as determined by your healthcare provider. Early breast cancer is when cancer is found only in the breast or nearby lymph nodes and has not spread to other parts of the body.
Caption: HR+, HER2–, hormone receptor positive, human epidermal growth factor receptor 2 negative, node-positive, early breast cancer with a high risk of coming back as determined by your healthcare provider. Verzenio is given with hormone therapy.
00:39-00:54
[On the animated cell diagram, the CDK4 & CDK6 proteins are labeled. The cell membrane, nucleus, hormone receptors and estrogen hormones are labeled on the normal breast tissue animated cell diagram.]
AVO: Verzenio works to block specific proteins called CDK4 and CDK6. CDK4 & 6 proteins help control how quickly cells grow. These proteins, along with hormones like estrogen, play an important role in the growth and division process of normal cells.
Cell diagram label: CDK4 & CDK6 proteins.
Cell diagram label: Normal breast tissue cell. Estrogen hormones. Cell membrane. Hormone receptors. Nucleus.
00:55-1:09
[We see an animation of a "breast tissue cancer cell" with "overactive CDK4 & 6 proteins" labeled on the cell. The cancer cell divides into two cells.]
AVO: But if you have HR+, HER2– early breast cancer, CDK4 & 6 proteins become overactive, causing the cancer cells to grow and divide uncontrollably, which makes the tumor grow.
Cell diagram label: Breast tissue cancer cell.
Cell diagram label: Overactive CDK4 & 6 proteins.
01:10-01:42
[The animated cell diagram shows how Verzenio works inside the cell to inhibit the CDK4 & 6 proteins.]
AVO: Just as cancer cells prepare to divide, Verzenio works to block specific CDK4 & 6 proteins. Verzenio is a CDK4 & 6 inhibitor, used to treat HR+, HER2–, node-positive early breast cancer with a high risk of coming back. Verzenio is taken with a hormone therapy to help prevent cancer recurrence after active treatment. Hormone therapy works outside the cell to reduce estrogen that feeds the cancer. Verzenio works inside the cell to block CDK4 & 6 activity and help stop the growth of cancer cells.
Caption: Verzenio blocks CDK4 & 6 proteins.
Cell diagram label: CDK4 & 6 inhibitor. Verzenio. Hormone therapy works outside the cell. Verzenio works inside the cell.
01:43-01:58
[In the animated cell diagram, Verzenio is working inside to inhibit the CDK4 & 6 proteins. The Verzenio and Lilly logos appear on the bottom of the screen.]
AVO: When Verzenio inhibits CDK4 & 6 in healthy cells it can lead to side effects, some of which may be serious. After active treatment, such as surgery, chemotherapy and radiation, Verzenio is taken with hormone therapy to help prevent the cancer from returning.
Caption: Based on pre-clinical studies.
01:59-04:02
[The Verzenio logo is in the bottom right corner.]
AVO/Caption: INDICATION AND SAFETY SUMMARY
Verzenio® (ver-ZEN-ee-oh) is used to treat certain types of breast cancer known as HR+/HER2– (hormone receptor positive/human epidermal growth factor receptor 2 negative) breast cancer.
It is a medicine you can take if:
- You have node-positive early breast cancer that has a high risk of coming back as determined by your healthcare provider. Verzenio is given along with hormonal therapy to women and men.
It is not known if Verzenio is safe and effective in children.
Warnings - Verzenio may cause serious side effects, including:
Diarrhea is common with Verzenio, may sometimes be severe and may cause dehydration or infection. The most common time to develop diarrhea is during the first month of Verzenio treatment. If you develop diarrhea during treatment with Verzenio, your healthcare provider may tell you to temporarily stop taking it, stop your treatment, or decrease your dose.
If you have any loose stools, start taking an antidiarrheal medicine (such as loperamide), drink more fluids, and tell your healthcare provider right away.
Low white blood cell counts (neutropenia) are common with Verzenio and may cause serious infections that can lead to death. Your healthcare provider should check your white blood cell counts before and during treatment. If you develop low white blood cell counts during treatment with Verzenio, your healthcare provider may tell you to temporarily stop taking it, decrease your dose, or wait before starting your next month of treatment. Tell your healthcare provider right away if you have signs and symptoms of low white blood cell counts or infections, such as fever and chills.
Verzenio may cause severe or life-threatening inflammation (swelling) of the lungs during treatment that can lead to death. If you develop lung problems during treatment with Verzenio, your healthcare provider may tell you to temporarily stop taking it, decrease your dose, or stop your treatment. Tell your healthcare provider right away if you have any new or worsening symptoms, including:
- Trouble breathing or shortness of breath
- Cough with or without mucus
- Chest pain
04:03-05:56
[The Verzenio logo is in the bottom right corner.]
Caption: INDICATION AND SAFETY SUMMARY
AVO/Caption: Verzenio can cause serious liver problems. Your healthcare provider should do blood tests to check your liver before and during treatment. If you develop liver problems during treatment with Verzenio, your health care provider may reduce your dose or stop your treatment. Tell your healthcare provider right away if you have any of the following signs or symptoms of liver problems:
- Feeling very tired
- Pain on the upper right side of your stomach area (abdomen)
- Loss of appetite
- Bleeding or bruising more easily than normal
Verzenio may cause blood clots in your veins, or in the arteries of your lungs. Verzenio may cause serious blood clots that have led to death. If you develop blood clots during treatment with Verzenio, your healthcare provider may tell you to temporarily stop taking it. Tell your healthcare provider right away if you have any of the following signs and symptoms of a blood clot:
- Pain or swelling in your arms or legs
- Shortness of breath
- Chest pain
- Fast breathing
- Fast heart rate
Verzenio can harm your unborn baby. Use effective birth control (contraception) during treatment and for 3 weeks after the last dose of Verzenio and do not breastfeed during treatment with Verzenio and for at least 3 weeks after your last dose. Verzenio may affect the ability of males to father a child.
Common side effects
The most common side effects of Verzenio include:
- Nausea
- Infections
- Low red blood cell counts (anemia)
- Decreased appetite
- Headache
- Hair thinning or hair loss (alopecia)
- Abdominal pain
- Tiredness
- Low white blood cell counts (leukopenia)
- Vomiting
- Low platelet counts (thrombocytopenia)
These are not all the possible side effects of Verzenio.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
05:57-07:08
[The Verzenio logo is in the bottom right corner.]
Caption: INDICATION AND SAFETY SUMMARY
AVO/Caption: Before using
Before you use Verzenio, tell your healthcare provider about all your medical conditions, including:
- If you have fever, chills, or other signs of infection.
- If you have a history of blood clots in your veins.
- Have lung or breathing problems.
- Have liver or kidney problems.
- If you are pregnant, plan to become pregnant, or are breastfeeding.
- About all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take a medicine that contains ketoconazole.
How to take
- Take Verzenio exactly as your healthcare provider tells you.
- Your healthcare provider may change your dose if needed. Do not stop taking Verzenio or change the dose without talking to your healthcare provider.
- Verzenio may be taken with or without food.
- Swallow Verzenio tablets whole. Do not chew, crush, or split the tablets before swallowing. Do not take Verzenio tablets if they are broken, cracked, or damaged.
- Take your doses of Verzenio at about the same time every day.
- If you vomit or miss a dose of Verzenio, take your next dose at your regular time. Do not take 2 doses at the same time to make up for the missed dose.
07:09-08:08
What to avoid during treatment
- Avoid taking ketoconazole during treatment with Verzenio. Tell your healthcare provider if you take a medicine that contains ketoconazole
- Avoid grapefruit and products that contain grapefruit during treatment with Verzenio. Grapefruit may increase the amount of Verzenio in your blood
[The Verzenio logo is in the bottom right corner.]
AVO/Caption: INDICATION AND SAFETY SUMMARY
Learn more
Verzenio is a prescription medicine. For more information, call 1-800-545-5979 or go to verzenio.com.
This summary provides basic information about Verzenio but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your healthcare provider. Be sure to talk to your healthcare provider about Verzenio and how to take it. Your healthcare provider is the best person to help you decide if Verzenio is right for you.
AVO/Caption: Verzenio® is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries or affiliates.
Caption: AL CON BS_mE 14SEP2022
PP-AL-US-3852 08/2023 ©Lilly USA, LLC 2023. All rights reserved.
08:09-08:22
[The Verzenio and Lilly logos are center screen.]
AVO: Talk to your doctor if you have any other questions about Verzenio.
Caption: Talk to your doctor about Verzenio.
For pricing information, please visit lillypricinginfo.com/verzenio or call 1-844-VERZENIO (1-844-837-9364).
PP-AL-US-3852 08/2023 ©Lilly USA, LLC 2023. All rights reserved.
Verzenio® is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries or affiliates.
Learn about Savings & Support, including how to enroll in the Verzenio Continuous Care™ Program