
Verzenio + fulvestrant
Explore the effectiveness and dosing of Verzenio + fulvestrant.
How effective is Verzenio + fulvestrant?
Verzenio was studied in a clinical trial of 669 women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 negative (HER2–) metastatic breast cancer (MBC), whose disease had progressed after hormone treatment: 446 patients were treated with Verzenio + fulvestrant, and 223 were treated with fulvestrant alone.
Verzenio is helping to raise the bar of what is possible in HR+, HER2– metastatic breast cancer treatment.

Proven to help women live significantly longer
In a clinical study, women taking Verzenio + fulvestrant lived for a median of 46.7 months vs 37.3 months on fulvestrant alone, regardless of menopausal status.

Live longer without disease progression
In the same study, Verzenio + fulvestrant delayed disease progression for a median of 16.4 months vs 9.3 months with fulvestrant alone.

What is overall survival?
Overall survival (OS) is how long someone lives with cancer, either after being diagnosed or starting treatment. When taking treatment, OS is the days, months, or years that treatment may add to life.

What is progression-free survival?
Progression-free survival (PFS) is the amount of time during and after treatment that the cancer doesn't get worse. In other words, PFS is how long cancer growth is delayed from the time a treatment is started.
What is the difference between progression-free survival and overall survival?
Progression-free survival (PFS) is the amount of time during and after treatment that the cancer doesn't get worse. Overall survival (OS) is how long someone lives with cancer after being diagnosed or starting treatment.
SELECT SAFETY INFORMATION
Verzenio may cause serious side effects, including:
Diarrhea is common with Verzenio, may be severe and may cause dehydration or infection. The most common time to develop diarrhea is during the first month of Verzenio treatment. If you develop diarrhea during treatment, your healthcare provider may tell you to temporarily stop taking it, stop your treatment, or decrease your dose. If you have any loose stools, start taking an antidiarrheal medicine (such as loperamide), drink more fluids, and tell your healthcare provider right away.
Twice as many women saw their tumors shrink
48.1% of patients on Verzenio + fulvestrant saw their tumors shrink vs 21.3% with fulvestrant alone.

Complete response: no detectable signs of cancer
In a clinical study, 3.5% of women on Verzenio + fulvestrant had a complete response, or no detectable sign of cancer, vs 0% with fulvestrant alone.

Partial response: tumor shrinkage of 30% or more
In a clinical study, 44.7% of women on Verzenio + fulvestrant had a partial response with tumor shrinkage of 30% or more vs 21.3% with fulvestrant alone.
SELECT SAFETY INFORMATION
Verzenio may cause serious side effects, including:
Low white blood cell counts (neutropenia) are common with Verzenio and may cause serious infections that can lead to death. Your doctor should check your white blood cell counts before and during treatment. Tell your doctor right away if you have fever or chills.
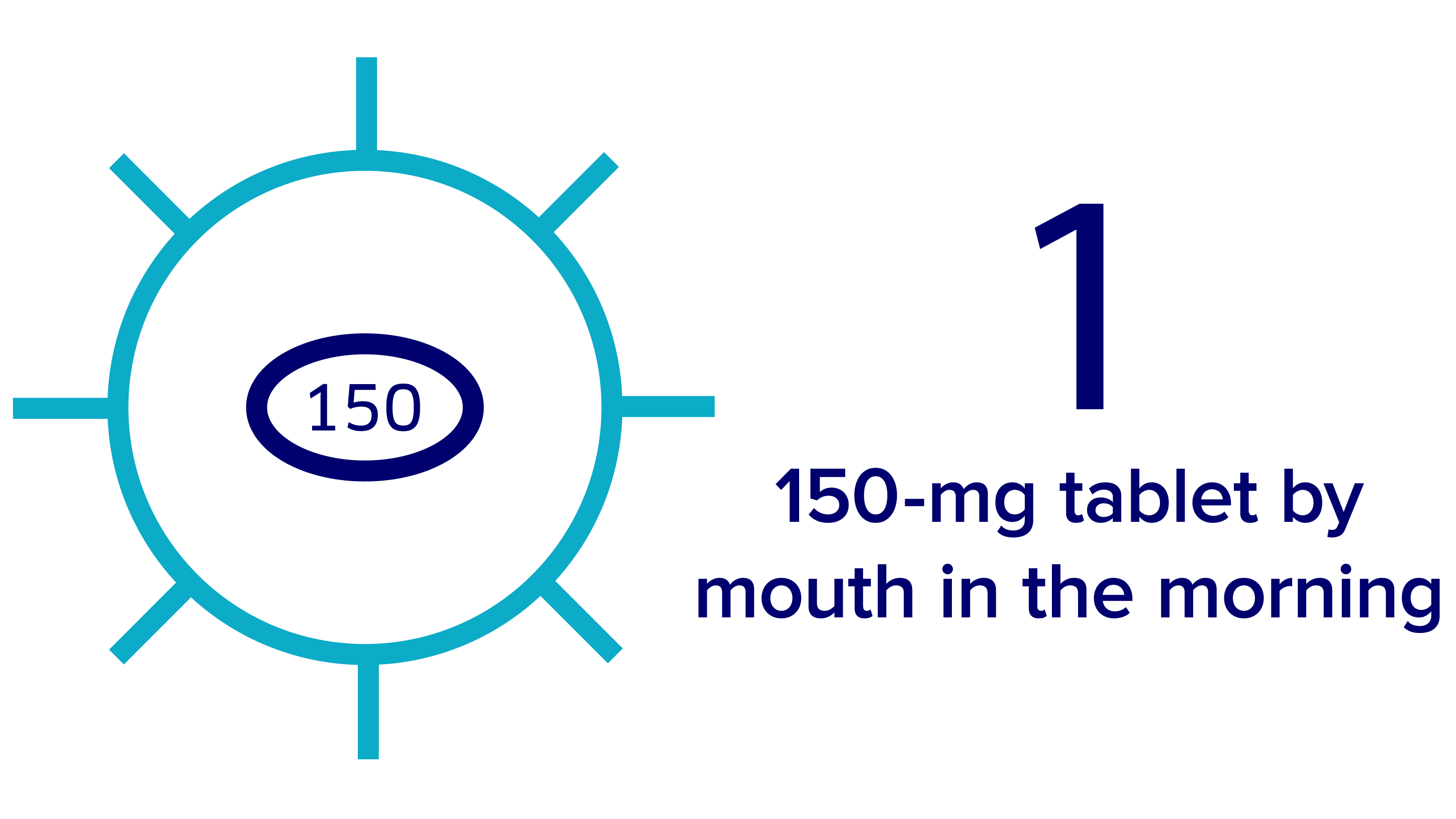
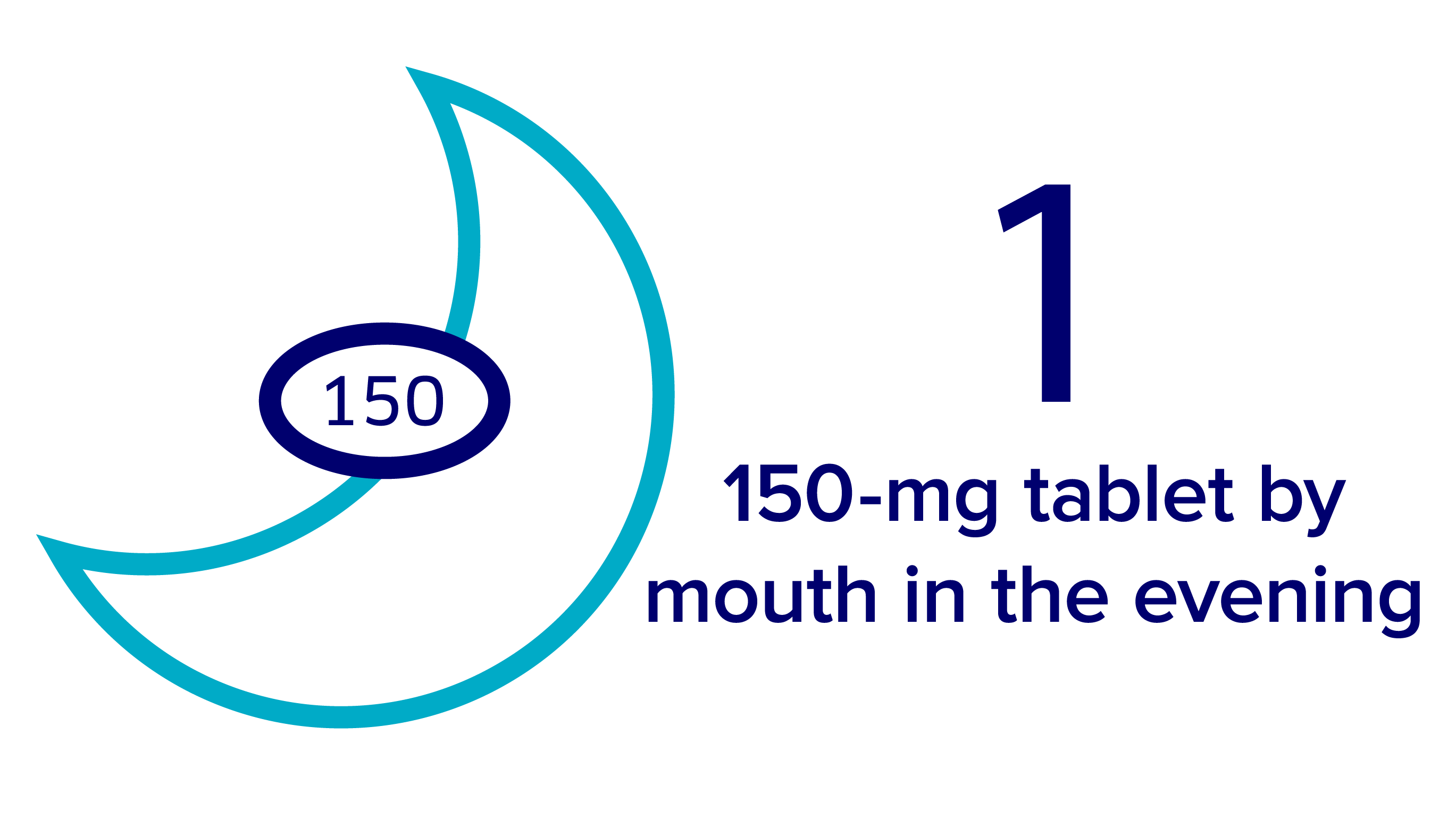
Taking Verzenio + fulvestrant
The recommended dose for Verzenio when given with fulvestrant is 150 mg twice daily, taken once in the morning and once at night.
You will receive 4 individual packs, each one containing your dose of 150-mg tablets for 7 days.
- Multiple tablet strengths (50 mg, 100 mg, and 150 mg) allow your doctor to make dose adjustments based on your tolerability
- If you need to reduce your dose due to tolerability, Verzenio is approved to be dosed as low as 50 mg twice daily, as directed by your doctor
- No matter what dose you are prescribed, you will take 1 tablet, 2 times a day, unless your doctor tells you otherwise
If your doctor prescribes:

50-mg dose
You will receive
4 red packs.

100-mg dose
You will receive
4 purple packs.

150-mg dose
You will receive
4 light blue packs.
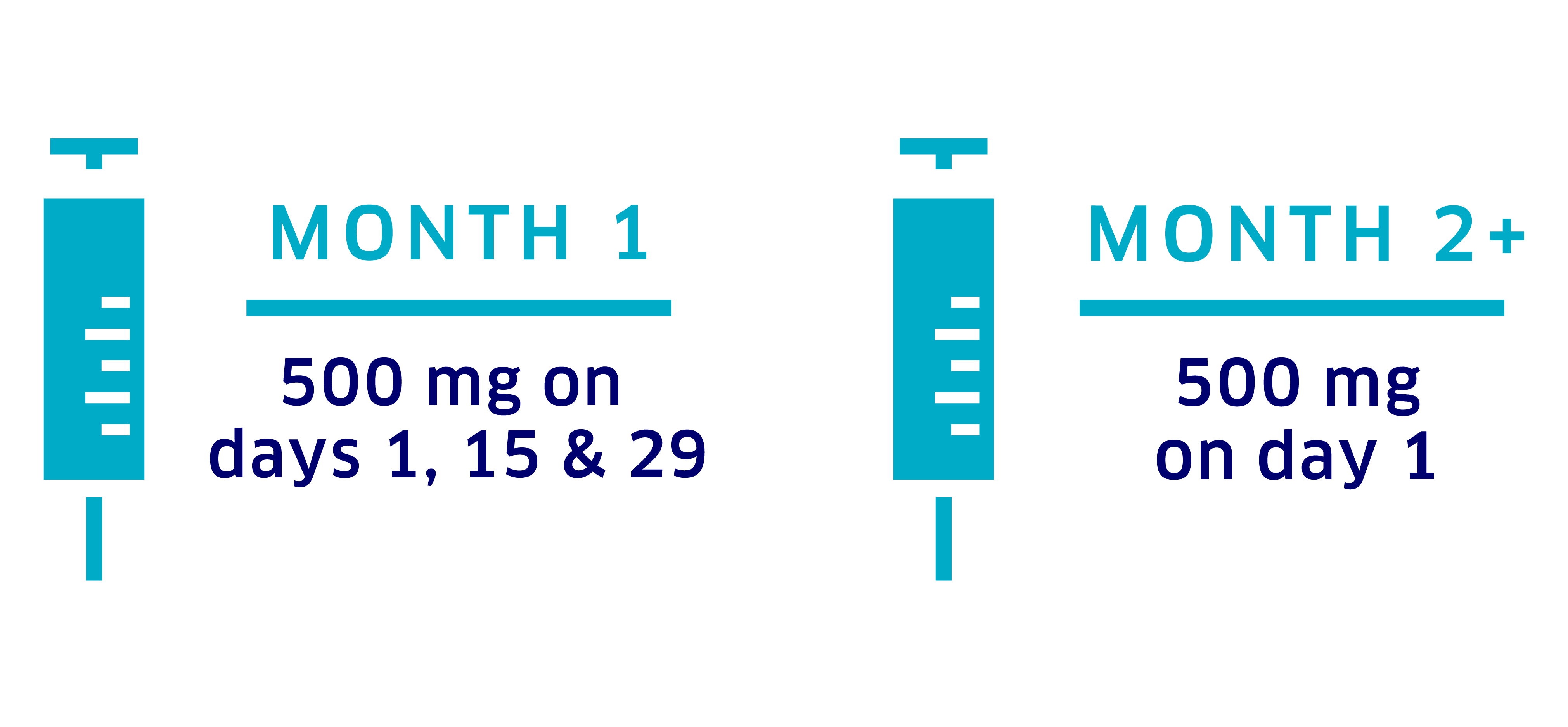
Fulvestrant dosing
The recommended starting dose of fulvestrant, when given with Verzenio, is 500 mg administered on days 1, 15, and 29 for the first month and once monthly thereafter. Your doctor will determine your fulvestrant dosing schedule.
If you have questions about Verzenio, or need more information on how to take it, you should talk to your doctor. You can also call us at 1-844-VERZENIO (1-844-837-9364) or sign up for our Verzenio Continuous Care™ Support Program.
Addressing side effectsLearn about Savings & Support, including how to enroll in the Verzenio Continuous Care Program